PTFE Creep Explained: Why Deformation Happens
If you’ve studied materials science, there’s one term you can’t avoid—PTFE creep.
Have you ever noticed plastic parts deforming slowly over time? Or found that a PTFE gasket, which fit perfectly when installed, sinks or compresses months later?
Is it just because the material is “soft”? Or is there a deeper molecular structure issue causing this slow, irreversible deformation?

In this article, we’ll use Polytetrafluoroethylene (PTFE) as an example and break down the concept of creep—from the electronic structure at the atomic level to the visible deformation at the macro scale. We’ll answer these key questions:
- What is the true nature of PTFE creep?
- Why does PTFE feel so “slippery”?
- How do molecular interactions and electron clouds play a role?
- How can we scientifically reduce or prevent creep?
What Is PTFE Creep?
Creep is the slow, irreversible deformation of a material under a constant load over time.
It’s not elastic deformation or brittle failure—it’s more like dough being slowly reshaped by the passage of time.
For example, when you tighten a PTFE gasket with a screw and revisit it months later, you may find that the contour has sunk. This is not an installation error—it’s the result of molecular chains gradually sliding under the combined effects of temperature and pressure.

Why Is PTFE So Slippery?
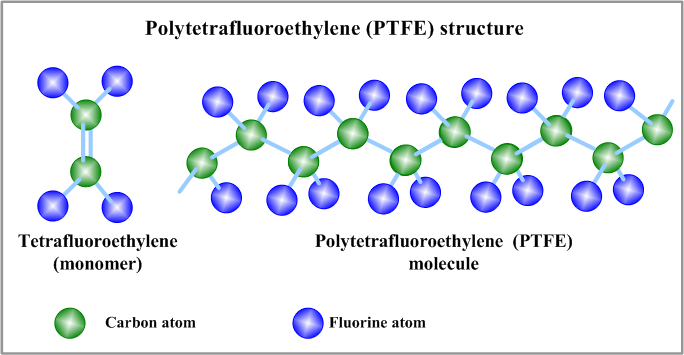
PTFE (polytetrafluoroethylene) has a highly regular molecular backbone made of –C–C– chains, with each carbon atom fully surrounded by fluorine:
Basic repeating unit: –[–CF₂–CF₂–]–
This structure leads to three key characteristics:
- Fully fluorinated – Fluorine atoms dominate the chain
- Highly non-polar – Little to no dipole interaction
- Linear chains – The molecules are straight and smooth

These properties mean that PTFE chains hardly “stick” to each other. Unlike other polymers, PTFE lacks:
- Hydrogen bonds (like PA or PVA)
- Dipole-dipole interactions (like PES)
- π–π stacking (like PI)
Instead, PTFE chains rely on van der Waals forces, which are inherently weak—and in PTFE’s case, even weaker than usual.
Why Are PTFE’s van der Waals Forces So Weak?
The answer lies in the nature of fluorine atoms:
- Small atomic radius (0.64 Å)
- Extremely high electronegativity (3.98—the highest of all elements)
- Densely packed electron clouds with extremely symmetric distribution
As a result, the electron clouds are difficult to polarize, which means PTFE molecules can’t form strong instantaneous dipoles. The weak van der Waals forces make PTFE chains behave like polished marble surfaces—nothing really sticks, and everything slides.
This extreme molecular-level freedom eventually manifests as macro-level creep.
Why Does This Lead to Creep?
At its core, polymer creep is the result of molecular chain segments slowly sliding past each other.
In PTFE, because the interchain resistance is so weak, even small amounts of heat or mechanical stress can trigger these slides. Over time, this results in visible deformation.
This sliding happens not continuously but in discrete jumps:
- Each chain segment must overcome an energy barrier
- When thermal energy or stress is sufficient, it “jumps” to a new state
- This is known as phonon-assisted transitions in quantum mechanics
In short: PTFE creep is the result of thermally activated “quantum jumps” of molecular segments. Since the barrier between chains is so low, they slide easily when heated.

Application in Instruments: PTFE as a Corrosion-Resistant Coating
PTFE is widely used as a protective coating in industrial instruments, such as Jiwei’s corrosion-resistant tuning fork level switch (as shown in the image). While its chemical inertness is excellent, creep must be considered in high-temperature or high-load environments.

How to Minimize Creep in PTFE?
To prevent or reduce creep, the core strategy is to limit molecular chain movement:
- Add fillers or crosslinking agents
Glass fiber or carbon fiber can significantly improve rigidity, reduce chain mobility, and enhance interchain interactions. - Control temperature and stress
The higher the temperature, the faster the creep. Although PTFE can withstand 260°C briefly, it’s best to keep long-term usage below 180°C to minimize deformation risk. - Design multilayer composite structures
Use PTFE as an intermediate or outer layer, with supporting materials on the outside to physically limit deformation and delay creep.
Conclusion
The extreme slipperiness of PTFE comes from ultra-weak van der Waals forces between its chains—due to highly symmetrical and tightly bound electron clouds on fluorine atoms. This molecular-level freedom leads to macro-level creep over time.
To use PTFE effectively, one must understand its molecular behavior and apply intelligent design and environmental control to mitigate the effects of creep.
